Semester 1, Academic Year 2001/2002 Section C
Question 16
Acetone is to be recovered from an acetone-air mixture by counter-current scrubbing with water in a packed tower. The inlet gas mixture has 6 mole % acetone. The total inlet gas flow rate is 0.8 kg/m2s (MW = 29) and the total inlet pure water flow rate is 1.0 kg/m2s (MW = 18).
The overall mass transfer coefficient KYa may be taken as 0.0164 kg-mole/(m3.s.mole fraction). The equilibrium solubility relationship for the system can be described by Henry's Law as y = 1.23 x.
What should be the height of the tower to remove 90% of the entering acetone
( 20 marks )
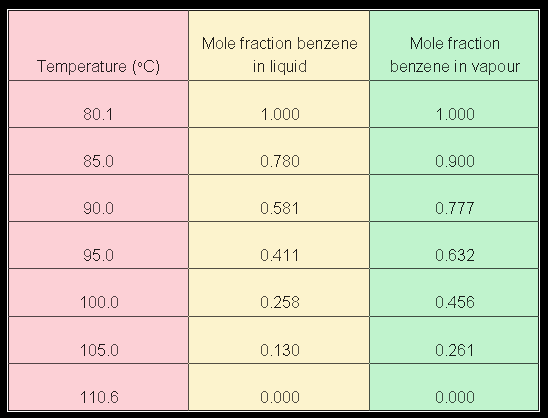
The following equations may be used: (subscript '1' refers
to conditions at the bottom of the tower, and subscript '2' refers to conditions
at the top of the tower; the other symbols have their usual meanings)
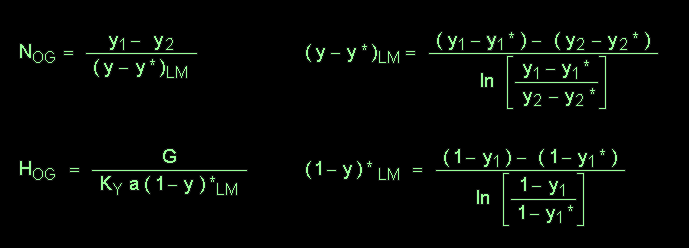
Question 17
An equimolar mixture of benzene and toluene is to be separated using continuous distillation into a top product containing 90 mole% benzene and a bottom product containing 90 mole% toluene. The distillation is to be carried out at atmospheric pressure. The total feed flow rate is 180 g-mole/hr and its q-value is 1.5.
Determine the following:
(a) The molar flow rates of distillate and bottoms products.
( 5 marks )
(b) The minimum reflux ratio.
( 7 marks )
(c) The number of theoretical trays required if a reflux of 1.5 times the minimum is used.
( 6 marks )
(d) The number of actual trays needed if the overall tray efficiency is 0.85.
( 2 marks )
Equilibrium data for benzene-toluene system at atmospheric pressure are given
below: