How does Distillation Works?
Liquid is first introduced to the bottom of the distillation column.
Steam is then introduced into the reboiler which heats up the mixture at the bottom of the column. The mixture is converted partially into vapour. The vapour so formed at the bottom of the column is richer in low boiling component of the binary mixture (in this case, ethanol) than the unvaporised liquid. The vapour is returned to the column and rises up the column.
To increase the concentration of ethanol in the vapour, the distillation column is equipped with trays or packings where the rising vapour will pass through. The vapour passes up the column and is completely condensed by a condenser located at the top of the column. For more information on trays and packing, check out the PLANT OPERATION & MONITORING Section.
The condensed liquid is then collected in the overhead accumulator, in which a definite liquid level is maintained. Some of the liquid in the accumulator is allowed to flow back to the top of the column. This liquid stream is called the external reflux or in short, just reflux. The reflux liquid is usually at its boiling point (in other words, it is a saturated liquid) and is rich in the low-boiling component (ethanol). It provides the down-flowing liquid that comes into contact with the up-flowing vapour.
The remaining portion of the condensed liquid is withdrawn as overhead product (i.e. the distillate).
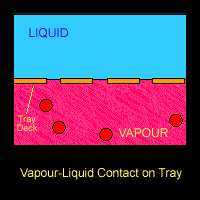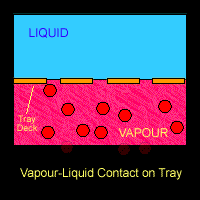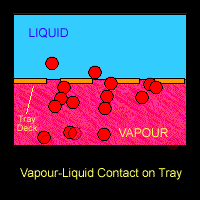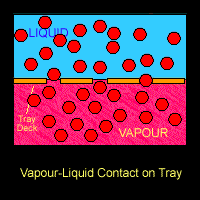
On the trays or around the packings where the vapour and liquid came into contact, mass transfer takes place. Lower boiling point component (ethanol) from the liquid going down the column will be vaporized and transferred into the vapour that is going up the column. On the other hand, the higher boiling point component (water) from the rising vapour will be condensed into the down-flowing liquid. Hence at each stage, the vapour is enriched in the lower-boiling component (ethanol) and the liquid is enriched in the higher boiling component (water).


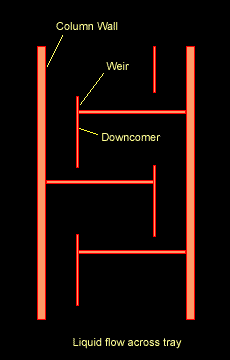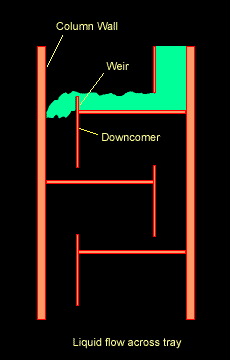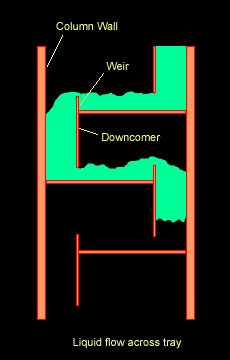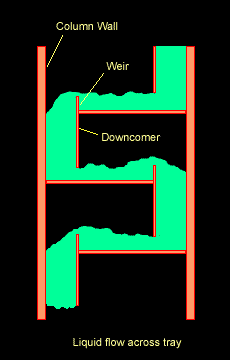
The Figure below shows a schematic diagram of vapour-liquid contact and the resultant mass transfer between the phases.
Hence, by means of mass transfer processes at each stage, nearly pure lower-boiling component (ethanol) can be obtained at the top of the column as overhead product and nearly pure higher-boiling component (water) at the bottom of the column as the bottoms product stream.
Other Important Points:
Vapour and liquid rates in the rectifying section need not be equal to the vapour and liquid rates in the stripping sections. This will be covered in greater details later.
For the case of a total condenser, concentration of the more volatile component is the same in the distillate, reflux and vapour leaving the top of the column.
The highest temperature and pressure occur at the bottom, lowest at the top.